How to Use
DEFINITY® RT
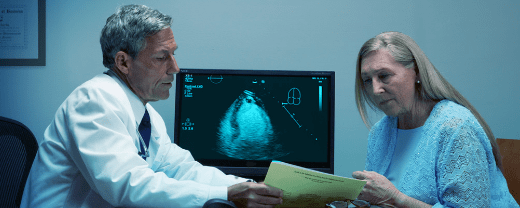
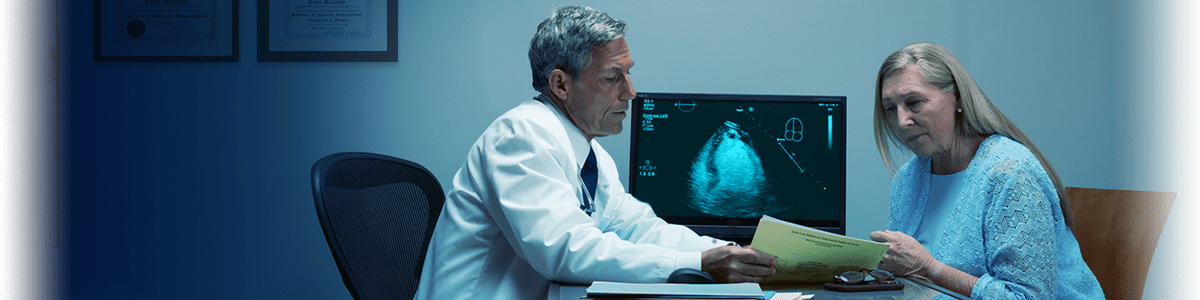
DEFINITY® RT Preparation Overview
Store at Room Temperature (68°‑77°F; 20°‑25°C)

Activate in VIALMIX® RFID
Attach ViaLok® Add 1.4 mL preservative-free Sodium Chloride Injection, USP
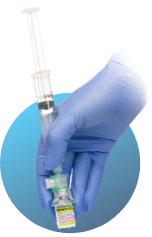
Swirl Rapidly 10 seconds
How to Activate DEFINITY® RT

- Activate DEFINITY® RT with VIALMIX® RFID
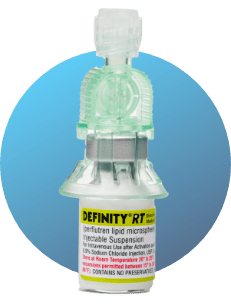
- Once activated, DEFINITY® RT will appear as a viscous, white solution in the vial
How to Prepare DEFINITY® RT
- Place the activated vial in the upright position and remove the flip-top cap. Insert 13 mm ViaLok® (Vented Vial Access Device) into the center of the rubber stopper and push down until properly locked onto the vial.
- Obtain an appropriate size syringe containing 1.4 mL preservative-free 0.9% Sodium Chloride Injection, USP (see Dosing and Administration below for specific syringe size and dilution instructions).
- Attach the syringe containing 1.4 mL preservative-free 0.9% Sodium Chloride Injection, USP to the ViaLok® luer-lok hub. Add 1.4 mL preservative-free 0.9% Sodium Chloride Injection, USP to the activated DEFINITY® RT vial. Do not inject air into the DEFINITY® RT vial.
- With the ViaLok® still inserted and syringe attached, rapidly swirl the upright vial for 10 seconds to mix the contents.
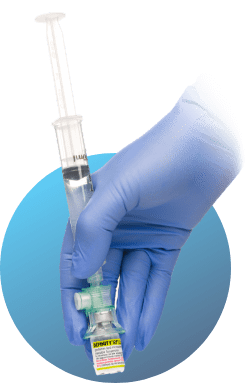
If the product is not used within 5 minutes of dilution, resuspend the microspheres by rapidly swirling the upright vial for 10 seconds before the product is withdrawn in the syringe.
Activated DEFINITY® RT may be used for up to 4 hours from the time of dilution, stored at room temperature in the product vial with the ViaLok® attached. A sterile syringe or cap should be attached to the ViaLok® until use. When ready to use, resuspend the microspheres by rapidly swirling the upright vial for 10 seconds before the product is withdrawn in the syringe.
DEFINITY® RT Dosing and Administration
DEFINITY® RT offers multiple dosing and administration options to meet patient- and practice‑specific needs.1
DEFINITY® RT Dosing Videos
DILUTED BOLUS
CONTINUOUS INFUSION
BOLUS
Choose Administration Method
Diluted Bolus
DEFINITY® RT mixed with preservative-free 0.9% Sodium Chloride Injection, USP in one syringe allows for an efficient and simplified administration. This saves time, avoids the need for a 0.9% Sodium Chloride Injection, USP flush, and eliminates use of additional supplies and waste.
- Use a 10 mL syringe filled with 10 mL preservative-free 0.9% Sodium Chloride Injection, USP, add 1.4 mL of the preservative-free 0.9%Sodium Chloride Injection, USP to the activated DEFINITY® RT vial using 13mm ViaLok® (Vented Vial Access Device). Rapidly swirl the upright vial for 10 seconds
- Invert vial and withdraw 1.3 mL DEFINITY® RT into the syringe and combine with the remaining preservative-free 0.9% Sodium Chloride Injection, USP
- Gently hand-agitate the syringe to evenly distribute microbubbles
- Administer ~2 mL slowly. Typically 10 seconds per mL with subsequent injections as needed
For more details on the diluted bolus method, watch this instructional video.
Continuous Infusion
A continuous flow of DEFINITY® RT combined with preservative-free 0.9% Sodium Chloride Injection, USP provides a consistent, steady enhancement. This method is well-suited for studies performed over an extended period.
- Use a 3 or 5 mL syringe filled with 1.4 mL preservative-free 0.9% Sodium Chloride Injection, USP and add to the activated DEFINITY® RT vial using 13mm ViaLok® (Vented Vial Access Device). Rapidly swirl the upright vial for 10 seconds
- Invert vial and withdraw 1.3 mL DEFINITY® RT into the syringe
- Combine the 1.3 mL DEFINITY® RT with a 50 mL IV bag containing preservative-free 0.9% Sodium Chloride Injection, USP
- Gently squeeze IV bag to evenly distribute microbubbles
- Initiate at 4 mL/minute; maximum 10 mL/minute
- Adjust flow rate for optimal image enhancement
For more details on the continuous infusion, watch this instructional video.
Bolus
DEFINITY® RT may be administered as a simple, straight bolus injection followed by a 0.9% Sodium Chloride Injection, USP flush to offer an easy, rapid image enhancement in small doses.
- Use a 3 mL syringe filled with 1.4 mL preservative-free 0.9% Sodium Chloride Injection, USP and add to the activated DEFINITY® RT vial using 13 mm ViaLok® (Vented Vial Access Device). Rapidly swirl the upright vial for 10 seconds
- Withdraw 10 μL/kg DEFINITY® RT into the syringe. Administer slowly over 30 to 60 seconds. Follow with a 10 mL 0.9% Sodium Chloride Injection, USP flush. Subsequent injection as needed
Smaller, incremental dose amounts of 0.2 mL to 0.3 mL are better suited for current ultrasound system technology. The maximum allowable dose is 20 μL/kg.
For more details on the bolus method, watch this instructional video.
References:
-
DEFINITY® RT [package insert]. N. Billerica, MA: Lantheus Medical Imaging, Inc.
-
VialMix® RFID User's Guide. N. Billerica, MA: Lantheus Medical Imaging, Inc.
-
Sboros V, Moran CM, Pye SD, McDicken WN. Contrast Agent Stability: A Continuous B-Mode Imaging Approach. Ultrasound in Med & Biol. 2001;27(10):1367-1377.


